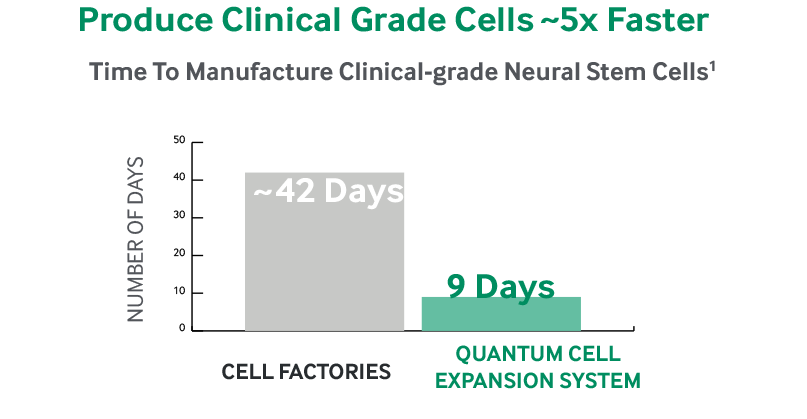
Cells | Free Full-Text | Development and Validation of a Good Manufacturing Process for IL-4-Driven Expansion of Chimeric Cytokine Receptor-Expressing CAR T-Cells

Stem-like memory T cells are generated during hollow fiber perfusion-based expansion and enriched after cryopreservation in an automated modular cell therapy manufacturing process - Cytotherapy

A Comparison of Automated Perfusion- and Manual Diffusion-Based Human Regulatory T Cell Expansion and Functionality Using a Soluble Activator Complex - Mark Jones, Brian Nankervis, Kelly Santos Roballo, Huong Pham, Jared Bushman,

Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum® Cell Expansion System for the culture of human mesenchymal stem cells - ScienceDirect
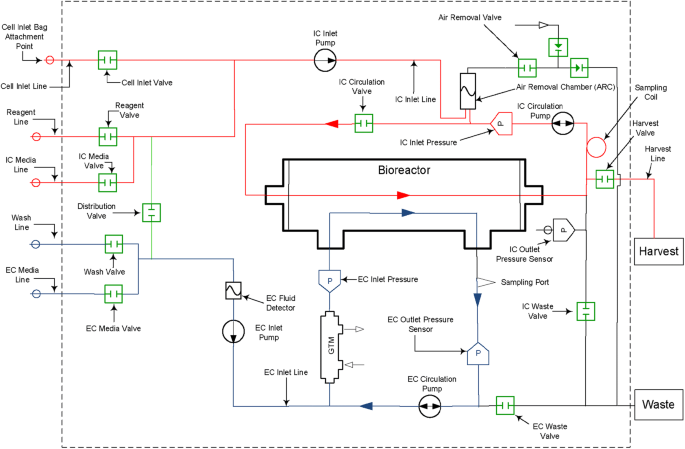
Large-scale expansion and characterization of CD3+ T-cells in the Quantum® Cell Expansion System | Journal of Translational Medicine | Full Text

Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum® Cell Expansion System for the culture of human mesenchymal stem cells - ScienceDirect
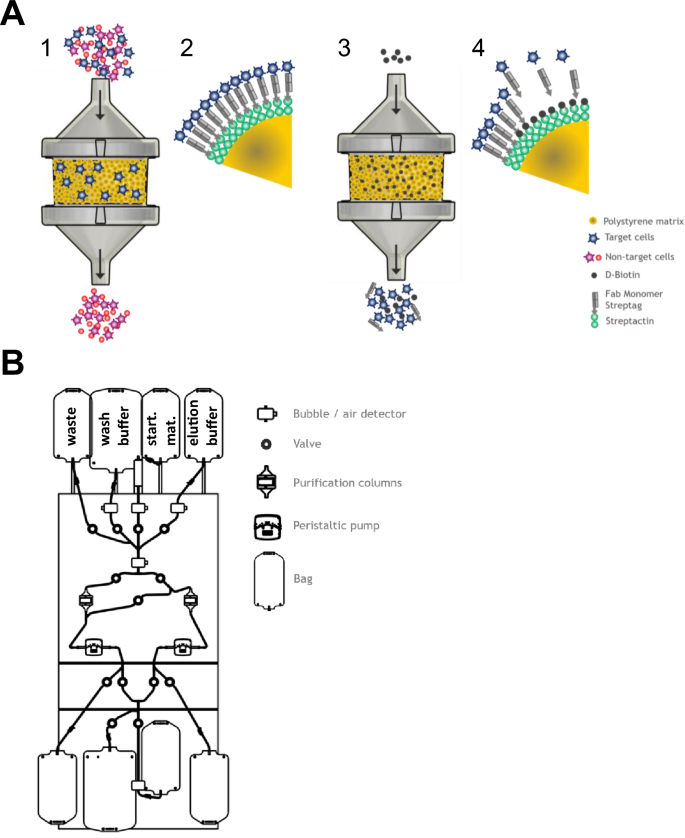
Next generation automated traceless cell chromatography platform for GMP-compliant cell isolation and activation | Scientific Reports

Frontiers | GMP-Compliant Production of Autologous Adipose-Derived Stromal Cells in the NANT 001 Closed Automated Bioreactor
Frontiers | Cell-Based Therapy Manufacturing in Stirred Suspension Bioreactor: Thoughts for cGMP Compliance
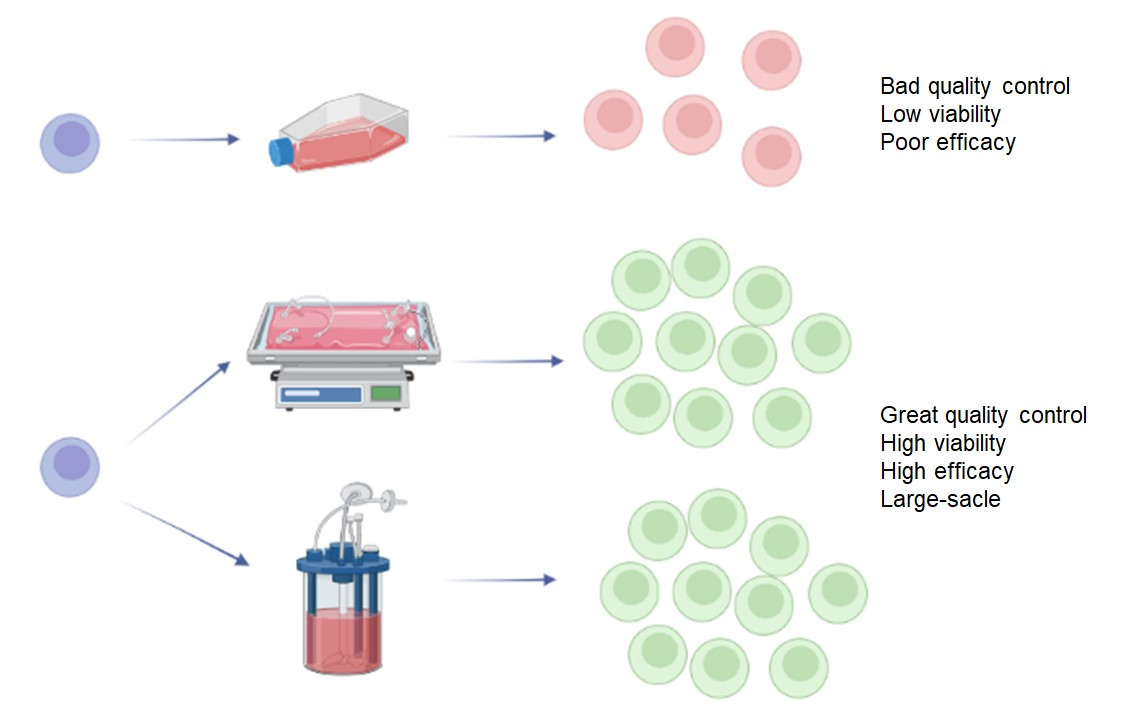
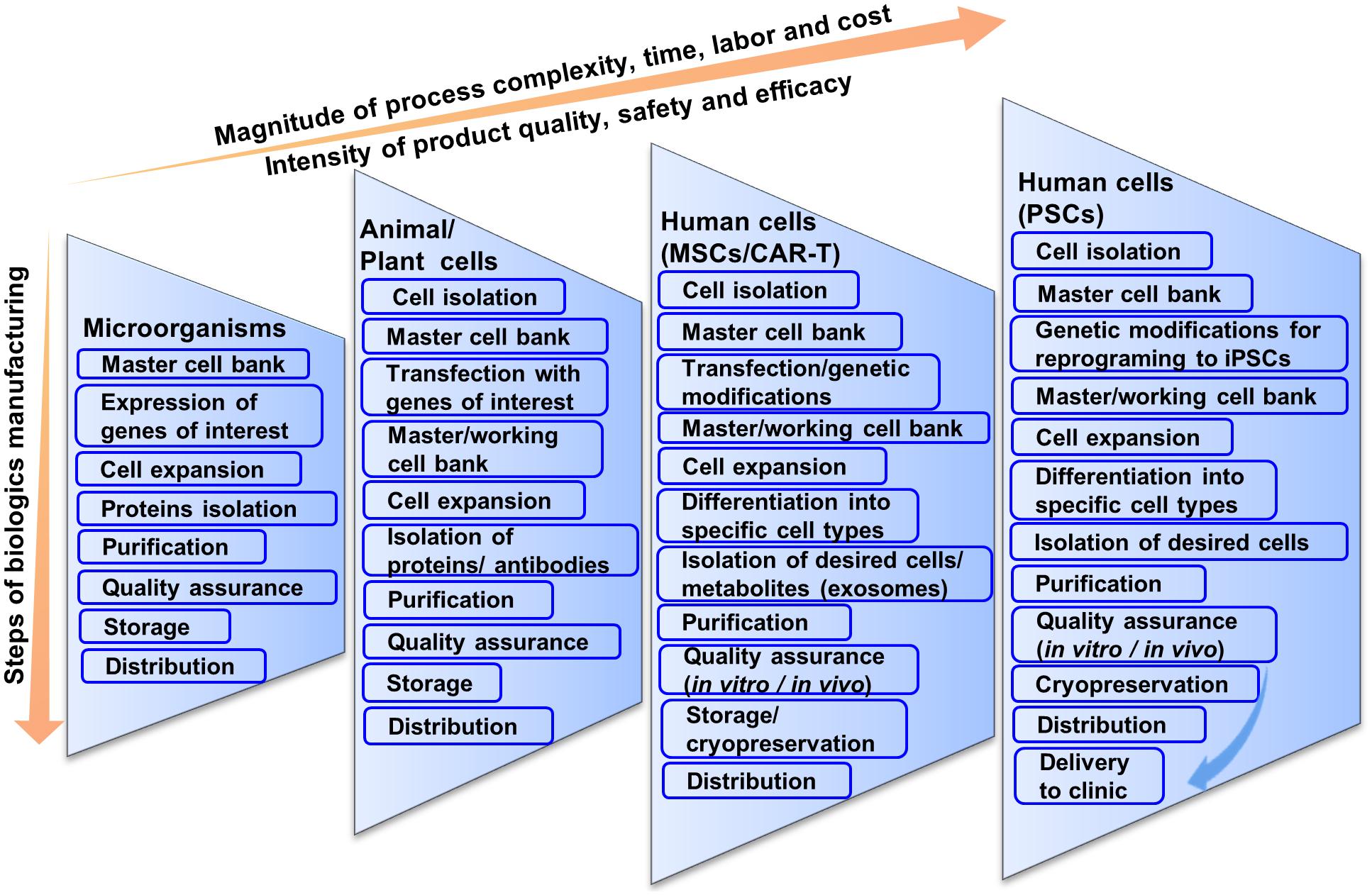
Bioengineering | Free Full-Text | Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies

The WAVE bioreactor and static bags expand the same lymphocyte cultures... | Download Scientific Diagram

Automated production of CCR5-negative CD4+-T cells in a GMP-compatible, clinical scale for treatment of HIV-positive patients | Gene Therapy